Core Nutritionals LOAD
$44.99
- GS4 Plus® – Effectively reduce measurements of plasma glucose
- Green Coffee Extract – Increase nutrient partitioning capacity of myocytes
- Berberis Aristata – Help support healthy blood glucose metabolism
- ✔ Percentage Reward Add this item to unlock your discount to get 30% off !
Out of stock
Physiological Properties and Effects:
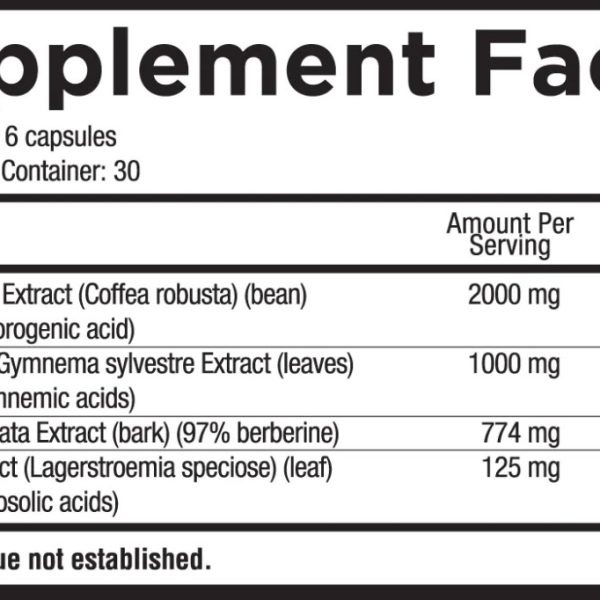
Green Coffee Extract (Coffea robusta) (bean) (50% Chlorogenic acid)
Coffee is widely recognized as not only the most consumed psychoactive compound on earth, but perhaps also the most consumed plant material in general. Due to the extent of coffee consumption by humans, several species of coffee beans, along with their bioactive constituents, have been research targets for a number of therapeutic pathways. The most abundant polyphenol in coffee is Chlorogenic acid (CGA). An abundance of data suggests that CGA has numerous physiological properties, and that its most potent physiological effects are hypoglycemic (blood sugar-lowering) and lipolytic/anti-lipogenic in nature. Recent evidence demonstrates that CGA is a powerful and novel glucose modulator, in vivo, in both healthy and diseased populations.
Research shows that one of CGA’s potential hypoglycemic mechanisms is insulin sensitization – in other words, making existing cells more sensitive to the glucose shuttling effects of insulin. In a trial on streptozotocin (STZ) (45 mg/kgbw) nicotinamide-induced diabetic rats, a 5mg/kgbw dose of CGA significantly mitigated the diabetic effects of STZ and caused an increase to glucose uptake. Of note, the 5mg/kgbw dose of CGA used in the animal trial is significantly less than the mg/kgbw serving size of CGA included in Core LOAD. (Assuming an average Core LOAD consumer bodyweight of 75kg.) An additional trial in obese Zucker rats demonstrated that CGA is capable of improving glucose tolerance and insulin resistance. The trial found that the CGA group had better glucose tolerance, higher insulin sensitivity index (ISI), and lower HOMA-IR (insulin resistance) index.
A trial in healthy humans showed similar promise. Healthy, human subjects were challenged with an acute serving of CGA and then assessed for hepatic glucose output, blood glucose levels, and glucose tolerance. CGA induced a significant reduction in peak plasma glucose levels – an important fact, as glucose fluctuations and/or chronically excessive blood glucose levels are associated with fat cell proliferation. Unlike the animal trial, however, the human trial hypothesized that CGA worked through a different, more novel mechanism: attenuating glucose absorption in the gastric uptake phase. In this trial, CGA effectively CGA functioned as both an insulin sensitizing agent and a glycemic index lowering agent.
In addition to both the above mechanisms, CGA was shown to inhibit the activities of α-amylase and α-glucosidase – two enzymes responsible for metabolizing carbohydrates into saccharides. As a result, CGA again reduced postprandial (post-meal) blood glucose concentration. The potentially novel glycemic index-lowering and carbohydrate metabolism effects of CGA were confirmed in two further trials: one in which CGA intake significantly reduced fasting glucose and insulin levels in obese men, and another in which CGA lowered the glycemic impact (how quickly and to what degree plasma glucose was increased) and lowered blood glucose levels.
CGA is an interesting compound due not only to its potent insulin-sensitizing and glycemic effects, but also its novel action on lipid metabolism. A 2006 trial found that CGA – in addition to caffeine and other polyphenols found in green coffee bean extract – suppressed body weight gain and the accumulation of visceral fat in mice. Again, the mechanism of action was quite unique: CGA was found to inhibit and delay the absorption of fat at the gastric uptake phase and reduce lipid metabolism in the liver.
In a separate trial on obese rates, CGA significantly reduced body weight, visceral fat mass (adipose tissue surrounding the internal organs), and plasma insulin concentrations. The authors of this trial hypothesize that, in addition to the profound effects on glucose metabolism noted above, CGA reduced body weight and fat mass due to increased fatty acid beta-oxidation activity and peroxisome proliferator-activated receptors alpha expression in the liver.
In sum, CGA is the perfect confluence of glycogenic, glycemic-impact lowering, insulin sensitizing, and anti-lipogenic factors. Its inclusion as a key ingredient in Core LOAD, at well above clinical serving sizes, functions to promote healthy blood glucose levels, healthy bodyweight, and healthy insulin release.
GS4 Plus® Gymnema sylvestre Extract (leaves) (25% gymnemic acids)
Gymnema sylvestre is a plant endemic to the tropical and sub-tropical regions of Southeast Asa, including India and China. In these countries, G. sylvestre functions as a treatment for many pathological conditions, including diabetes and hypercholesterolemia – in fact, the Ayurvedic name for G. sylevestre is “Gurmar,” or “destroyer of sugars.” The principle bioactives in G. sylvestre, gymnemic acids, are concentrated highly in the leaves and have been isolated and observed for their glucose shuttling properties.
In a clinical trial in humans using a G. sylestre extract dietary supplement, 65 participants were administered G. sylvestre for 90 days. After the 90 day treatment, the participants were assessed for a number of biometrics, including prenadial (pre-eating) plasma glucose concentrations and HbA1c (haemoglobin A1c, a key marker for diabetes) levels. The researchers found that 90 days of supplementation with G. sylvestre lowered prenadial blood glucose by 11%, postpranadial glucose by 13%, and Hb1Ac by .8% (a seemingly small figure, but in fact statistically significant).
In another, small clinical study, fasting blood glucose levels significantly improved in a group of type II diabetes mellitus patients after receiving a 200mg extract of G. sylevestre (standardized for gymnemic acid) for 20 months.
Unlike CGA, the mechanism(s) of action for G. sylevestre and gymnemic acid have not been precisely identified. Several trials in rats suggest that gymnemic acid’s structural similarity to glucose may have a role in its hypoglycemic effects; while still other trials suggest that, like CGA, gymemic acid may exert glucose uptake inhibitory effects at the intestinal level.
Whatever the mechanism of action, however, the plant has demonstrated the ability to promote healthy blood glucose levels and assist in the management of a healthy body mass.
Berbis aristata Extract (bark) (97% berberine)
Berberis aristata, is a shrub belonging to the family Berberidaceae and the genus Berberis, found in many parts of Eurasia and the Americas. The bark and bark extracts of the plant have been used traditionally as an antibacterial and antifungal preparation and as a hypoglycaemic, anti-diuretic, and vasodilator. The use of both B. aristata and its principal constituent, berberine, is widespread in traditional medicinal systems throughout history. Berberine is also now a prominent ingredient in dietary supplement formulations and a research target for numerous pathological conditions.
There is extensive literature demonstrating that the bark extract of B. aristata – and other plants containing a large proportion of berberine – is a potent AMPk agonist in skeletal muscle. Based on the pharmacological similarities between berberine and the anti-diabetic medicine metformin (with respect to AMPk activation), it is possible that berberine may promote an anti-catabolic state through increased expression of AMPk. In a randomized, placebo-controlled and double blind trial, patients with Type II diabetes mellitus risk factors were administered an oral preparation of 650mg Metformin for 8 weeks to assess body composition and changes in insulin resistance. The Metformin group experienced an increase in lean weight from 57.05 +/- 13.6 to 61.9kg +/- 16.5 kg (p < 0.01). Though the standard deviation suggests a large variation in lean tissue gain, the results are taken to be statistically significant at the 99th confidence interval, suggesting a high level of statistical precision. This indicates, in other words, that 99% of the study population experienced a lean weight increase. It should be noted that, in comparison studies, berberine extracts (at doses of 500-1500 mg/day) have outperformed metformin in key biochemical and physiological markers: including AMPk phosphorylation.
The human data – in both healthy and disease state populations – demonstrating berberine’s other physiological effects are numerous. A 2012 meta-analysis examined 14 randomized trials, containing 1068 patients, and assessed berberine’s efficacy in numerous biometrics, including resting plasma glucose levels, plasma insulin levels, body weight, body mass index, and reduction in fat mass and serum triglycerides and total cholesterol content. The aggregate data suggests that berberine performs at least as well as oral hypoglycemic agents (such as metformin) in these key metrics. Perhaps most interesting for users of Core LOAD, berberine showed the most promise in combination with oral hypoglycemic agents. While berberine lowered fasting plasma glucose and insulin levels, reduced HbA1c, reduced triglyceride levels and reduced fat mass alone, and performed well in comparison, it was shown to potentiate the effects of pharmaceutical anti-diabetics – at times significantly, with further reductions in the above metrics of up to 20%.
Effectively, this means that berberine is not only a potent glucose disposal agent on its own, but also makes other glucose disposal agents more effective.
Like CGA, berberine is not only a glucose disposal agent but a potent anti-lipidemic agent. In in vitro models, researchers examined the expression of a number of adipocyte-specific genes including fatty acid synthase (FAS), ADD1/SREBP1c (adipocyte determination and differentiation–dependent factor 1/sterol regulatory element–binding protein 1c), peroxisome proliferator–activated receptor (PPAR)γ, and 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1), and found them to be reduced in various WAT (white adipose tissue) deposits of berberine-treated mice. This data suggests that daily ingestions of beberine may potently inhibit a constellation of genes that are responsible for both depositing and converting lipids into fat tissue, and genes responsible for their mobilization (use for fuel) in response to exercise. In brown adipose tissue, the expression of PPARα mRNA (a receptor protein largely involved in lipolysis – the breakdown of fat tissue) also increased dramatically, while the mRNA of lipogenic genes such as FAS was reduced. Additionally, expression of PPARγ coactivator-1, a key regulator of several mitochondrial genes involved in adaptive thermogenesis, was substantially increased, suggesting berberine’s induction of AMPk expression leads to an inhibition of the proliferation and hypertrophy of fat cells.
Again, like CGA, berberine possesses numerous, beneficial physiological and metabolic properties: including the healthy regulation of blood glucose and accumulation of fat tissue. But perhaps most importantly, the evidence suggests that berberine may potentiate the effects of every other ingredient in Core LOAD.
Banaba Extract (Lagerstroemia speciose)(leaf)(20% corosolic acids)
Lagerstroemia speciosa is a flowering tree of the Lagerstroemia genus that is native to large portions of south east Asia, India and the Phillipines, and its bark and leaves have a historical tradition in folk medicine for the treatment of diabetes mellitus. Banaba (L. speciosa’s common name) has been used as a folk medicine to treat diabetes in various parts of the world, primarily southeast Asia. The hypoglycemic effect of aqueous (hot water) and methanol extracts have been demonstrated in many human studies. Most studies have focused on corosolic acid, the primary bioactive constituent of L. speciosa – and the compound for which Core LOAD’s banaba is standardized.
A 12-week lifestyle intervention study, involving 56 subjects consuming a dietary supplement containing banaba, taken prior to each meal, measured body mass at the conclusion of the trial. At the end of 12 weeks, the subjects had lost an average of 6.29 kg (13.8 lb) including 3.72 kg (8.2 lb) body fat as determined by a bioelectric impedance body fat analyser.
In a separate, smaller study, 12 nondiabetic subjects with a baseline blood glucose level of 104 mg/dL were given a soft gel capsule daily for 2 weeks containing 10 mg corosolic acid as a Banaba extract standardized to 18% corosolic acid. A 12% decrease in fasting glucose levels, as well as 60 min postprandial blood glucose levels, was observed after 2 weeks of administering the product. The authors also reported an average three-pound weight loss after the two weeks.
In an unpublished study using the same soft gel product containing 10 mg corosolic acid, 100 subjects with prediabetes or type 2 diabetes were enrolled. Half the subjects were given one soft gel containing the corosolic acid-standardized Banaba extract and the other half received a placebo for 30 days. Both fasting and 2 h postprandial blood glucose levels in the treated group decreased by 10% relative to the control (placebo) group. Also reported was an improvement in diabetic symptoms including a decrease in thirst, drowsiness, and hunger. Furthermore, no adverse effects were observed, with no changes in blood pressure, liver or kidney function, blood cell count, or hemoglobin.
Lastly, a study involving 31 subjects in a double-blind cross-over design were given a capsule containing 10 mg corosolic acid or a placebo 5 min before a 75g oral glucose tolerance test. Blood glucose levels were measured at 30 min intervals for 2h. The authors reported that corosolic acid treatment resulted in lower blood glucose levels from 60 to 120 min compared with controls, the difference being statistically significant (P < 0.05) at the 90 min time point. According to the authors, the corosolic acid that was used was 99% pure, thus indicating that the blood sugar lowering effect was specifically due to the corosolic acid. In the context of Core LOAD, the AUC (area under curve) demonstrated in this trial is important – as it demonstrates that corosolic acid exerts glucose stabilizing effects over a long time period (such as immediately before-to-after an intense workout).
The above clinical studies demonstrate that Banaba extract, Banaba extract standardized to corosolic acid, and corosolic acid itself, decrease fasting as well as postprandial blood glucose levels in humans. A decrease in blood glucose levels has been observed within 2 h of dosing, and the decrease is typically in the range of 10–15%, although a decrease of 30% has been reported.
The ability to corsolic acid to positively regulate healthy plasma glucose levels makes it a key addition to Core LOAD’s glucose disposal agent lineup.
Related products
General Health
General Health
General Health
Glucose Disposal
Glucose Disposal